The Edwards lab published a paper in the ACS journal Analytical Chemistry. The work, which was performed in collaboration with the laboratories of Prof. Julie Macpherson (Warwick, UK) and Prof. Henry S. White (Utah), takes a detailed look at how electrostatics influence the voltammetry of monolayers containing redox active headgroups. The work shows that charging and faradaic contributions to the current are intrinsically linked through the charge on the redox groups and that this should be taken into account when assessing electron transfer kinetics and surface coverage. For more details see: “Finite Element Modeling of the Combined Faradaic and Electrostatic Contributions to the Voltammetric Response of Monolayer Redox Films” (available as open access).
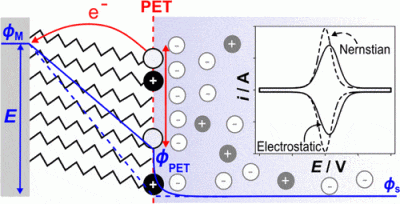
Schematic of a redox-active (O+/R) film on a metal electrode in contact with an electrolyte solution. The O+/R head groups define the plane of electron transfer (PET), which also includes neutral methyl spacers. The electric potential (ϕ) from the metal electrode across the film and into the bulk electrolyte is shown by the solid blue line. The potential drop between the PET and solution (ϕPET–ϕS) corresponds to the reduction in the driving force for electron transfer relative to a bare electrode.
Recent Comments